LENGTH-WEIGHT RELATIONSHIP AND CONDITIONAL FACTOR OF 16 IMPORTANT FISH SPECIES IN THE MEKONG DELTA, VIETNAM
Southern Branch of the Joint Vietnam-Russia Tropical Science and Technology Research Center
No.3, 3/2 street, ward 11, district 10, Ho Chi Minh City, Vietnam
Số điện thoại: +84974444119; Email: tvtien.itb@gmail.com
Nội dung chính của bài viết
Tóm tắt (Abstract)
The length-weight relationship (LWR) constitutes a fundamental analytical tool in ichthyological research, facilitating the assessment of growth dynamics, health status, and morphometric variations within fish communities. The present investigation was conducted to analyze the LWRs of economically and ecologically important fish species, which are predominantly exploited by small-scale fisheries within the Mekong Delta, Vietnam. Between January 2019 and March 2020, a total of 1,546 specimens, encompassing 16 species across 11 taxonomic families, were systematically collected. Morphometric data, specifically total length (TL) and body weight (W), were meticulously recorded for each individual, and sex was identified wherever feasible to enhance biological interpretation. The length-weight relationship was estimated using the equation W=a*TLb, where a and b represent species-specific constants. To enable statistical inference, a logarithmic transformation was applied, yielding a linear form: log(W) = log(a) + b*log(TL), which was subsequently analyzed using least-squares linear regression. All length-weight relationships deviations fell within the range of 2.72 to 3.28, of which eight species exhibited positive allometry (b > 3), seven species demonstrated negative allometry (b < 3), and one species showed isometric growth (b = 3). In addition, Fulton’s condition factor (K) was utilized as an indicator of fish health status. With the exception of two species from the genus Coilia, all estimated fish species showed comparatively high K-values, suggesting a generally favorable physiological condition among the studied fish populations. Collectively, these results highlight the overall health resilience and adaptive potential of fish communities inhabiting the dynamic aquatic habitats of the Mekong Delta.
Từ khóa (Keywords)
fisheries biology, condition factor analysis, allometric growth, isometric growth
Chi tiết bài viết
- Highlights:
Total of 1,546 fish specimens belonging to 16 species and 11 families were measured for total length and weight to estimate the length-weight relationship from January 2019 to March 2020.
The b coefficient of the length-weight relationship ranged between 2.72 and 3.28, of which eight species showed positive allometry, seven species exhibited negative allometry, and one species desmonstrated isometric growth.
Fulton’s condition factor ranged from 0.28 to 2.09 indicating that the healthy condition of fish in the Mekong Delta is satisfactory.
1. INTRODUCTION
Artisanal and subsistence fisheries in the lower Mekong basin provide a substantial amount of food and benefits to local communities, but most fish species are understudied, and data of their biological features are lacking [1]. In the Mekong River basin, the knowledge on fish has mostly focused on determining the species composition. Species richness is relatively well known and is estimated that approximately 400 fish species inhabit the different aquatic environments in the basin [2]. Nearly all species are harvested and used by the local population for commercial purposes or for their own consumption.
The length-weight relationship (LWR) and conditional factor (CF) are essential tools in fish biology research [3]. Obtaining length-weight relationships for species in multiple regions can be important in understanding intraspecific variability. LWR can also be used to document the reduction in fish size due to overfishing. For example, Halls et al. (2013) found no compelling evidence in fisheries monitoring data compiled in the lower Mekong basin to suggest that fish abundance, and mean fish size have declined significantly during the past 20 years [4].
The condition factor (CF) is a parameter used to assess the health and well-being of fish populations. The condition factor can offer insights into the overall health of a population, which is crucial for managing fishery strategies. A healthy fish population, indicated by a high condition factor, is often more sustainable. If fish exhibit a low condition factor, it may signal that the environment cannot support the current fishing levels or that fish are competing for limited resources, prompting a review of harvesting strategies to ensure sustainability.
This paper contributes to improving the biological information about length-weight relationships for fish species of different aquatic environments in the study area. The aim of this paper was: to find out the a and b values of LWR equations and conditional factors for 16 economically and ecologically important fishes [5, 6, 7, 8] of the Mekong Delta, Vietnam.
2. MATERIALS AND METHODS
Samples of fish were taken in the Mekong Delta (Vietnam) during the expedition survey in January 2019 and March 2020. The material was sampled from a fishing motor vessel using a towed fishing beam trawl with a rigid metal frame 4 m in width and 0.4 m in height; the length of the trawl bag was 12 m, and the mesh size of the net was 10 mm for the entire trawl. The trawling covered the entire delta, including two large rivers, namely, Hau (Bassac) and Tien (Mekong), and 8 estuarine branches (Figure 1). A total of 1,546 fish individuals from 16 species representing 11 families were collected. Fish were identified based on appropriate monographs comprising Rainboth (1996, 2012), Kottelat (2013) Tran et al. (2013) Taki et al. (2021) [2, 9, 10, 11, 12] and scientific names for each species were checked with FishBase [13]
The total length (TL) and weight (W) of each fish were measured. The sex of fish was also determined wherever possible. Immature samples were not used in the analysis. The length was estimated with an accuracy of 0.1 cm and the weight was measured with an accuracy of 0.1g. The length-weight relationship was estimated by using the equation: W=aTLb [14]. Parameters a and b of the LWR were estimated by linear regression analysis based on logarithms: log (W)=log (a) + b log (TL). To demonstrate the significant difference of obtained b-value in the equation from the isometric value 3, a t-test was used, expressed by the following equation: ts=(b-3)/s.e.b, where ts is the t-test value, b is slope and s.e.b – the standard error of slope (b). Comparison between obtained values of the t-test and the respective tabled critical values allowed the determination of the statistically significant b values, and their inclusion in the isometric range (b=3) or allometric range (negative allometric; b<3 or positive allometric; b>3). The drawing of the frequency distribution of the b-value with a superimposed normal curve was done using the ‘Frequencies’ routine (MS office Excel, 2019). Also, Fulton’s conditional factor (CF) was determined by the following equation: 100W/TL3 [3].
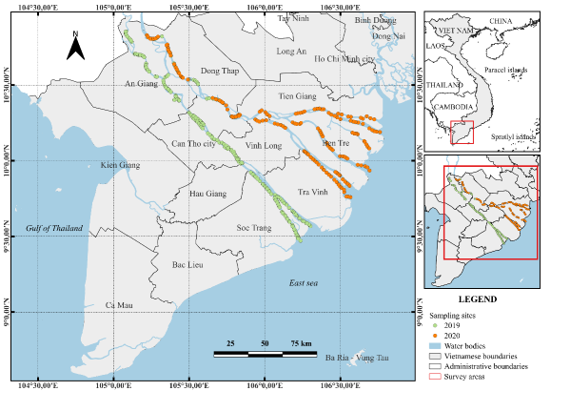
Figure 1. Surveying sites from 2019 to 2020 in the Mekong Delta, Vietnam
3. RESULTS AND DISCUSSIONS
3.1. Results
The results of LWR analyses of 16 fish in the Mekong Delta are summarized in Table 1. All regressions were highly significant, with the correlation coefficient (r) ranging from 0.88 to 0.99 (p<0.05). The frequency distribution of b values with a superimposed normal curve is shown in Figure 2. The mean value of b is estimated as 3.002, with a standard error of 0.042, i.e., the mean is close to 3, and 11 species had b close to 3 (isometric type of growth). The b values varied from 2.72 for Johnius trachycephalus (Bleeker, 1851) to 3.28 for Arius maculatus (Thunberg, 1792), of which 8 species had positive allometry (b>3), 7 species had negative allometry (b<3), and just one species isometry (b=3). The females of A. maculatus, Cephalocassis borneensis (Bleeker, 1851), Coilia lindmani Bleeker, 1857, Coilia rebentischii Bleeker, 1858, Parambassis wolffii (Bleeker, 1850), Polynemus melanochir Valenciennes, 1831 had higher b values than males. Female’s b values ranged from 2.77 for Clupeoides borneensis Bleeker, 1851 to 3.41 for A. maculatus and male’s b values ranged from 2.76 for P. wolffii to 3.15 for A. maculatus.
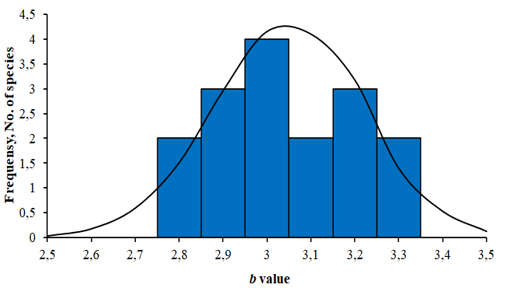
Figure 2. Frequency distribution of b value with superimposed normal curve from 16 fish species (both sexes) in the Mekong Delta (The distribution for the parameter b is close to normal. Isometric growth was observed in 4 species, negative allometric growth in 5, and positive allometric growth in 7).
The conditional factor for 16 fish species is shown in Table 1. The value of CF ranged from 0.28 for C. rebentischii to 2.09 for Datnioides polota (Hamilton, 1822). No significant sex differences in CF values were found for A. maculatus, Cl. borneensis, C. lindmani, and P. melanochir. Female of C. borneensis and P. wolffii had higher values of CF than male, C. rebentischii – opposite.
Table 1. Length-weight relationship and conditional factor of the fish species of the Lower Mekong Basin, Vietnam
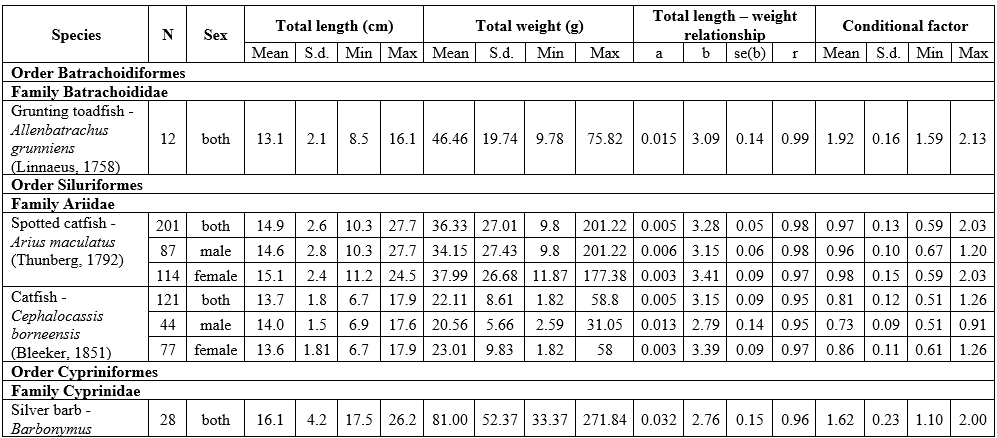
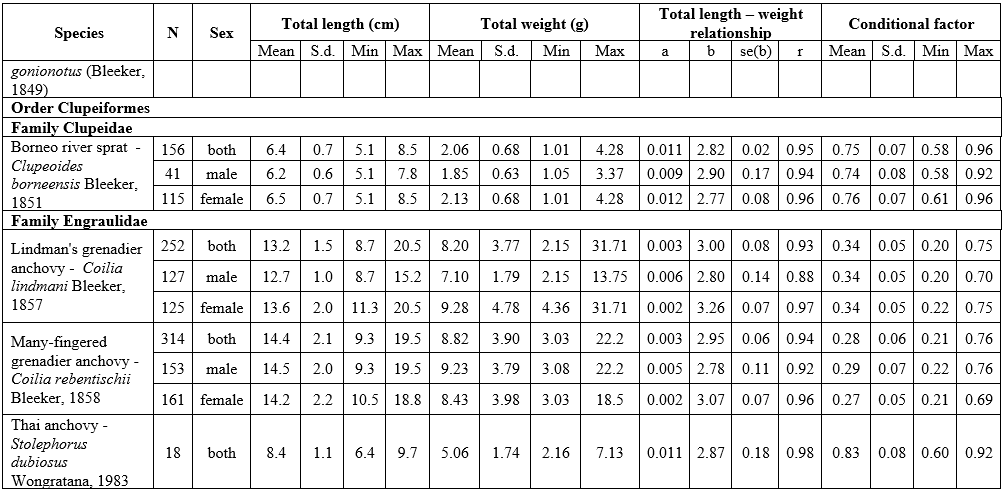
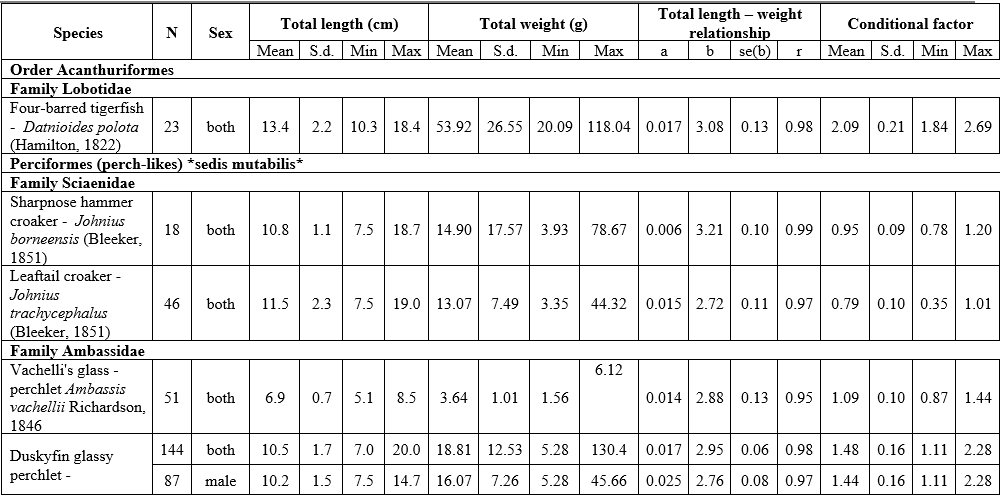
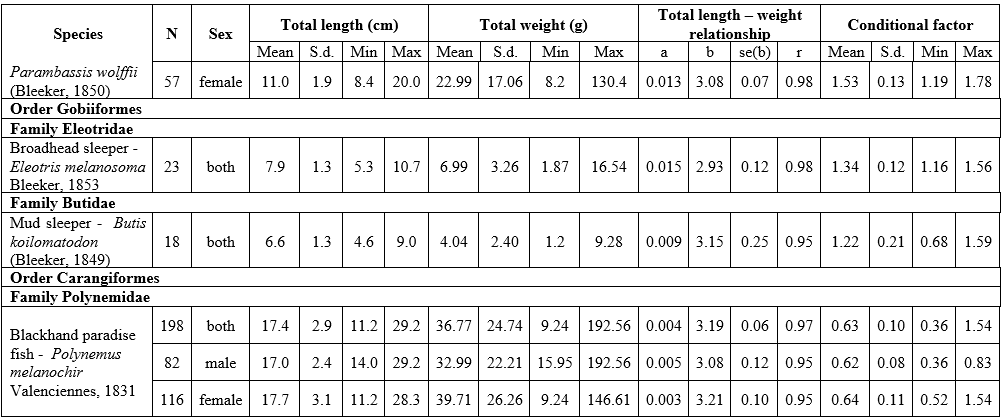
Abbreviations: N - number of individuals; S.d. – standard deviation
3.2. Discussions
Isometric growth occurs when an organism grows in such a way that its proportions remain constant. This type of growth typically implies that the body shape of the fish does not change as it grows. It is generally seen in species that have a stable environment and do not undergo significant changes in body shape throughout their life cycle. This might be advantageous in stable environments where predation or ecological niches do not vary significantly. Allometric growth occurs when different parts of the body grow at different rates. For example, a fish may grow longer while its weight increases at a different rate, leading to alterations in body shape. This is commonly observed in many fish species, where juvenile fish may have a different body morphology compared to adults. Allometric growth indicates adaptation to ecological pressures. A shift in body shape can help fish improve swimming efficiency, evade predators, or occupy different ecological niches as they mature. In many species, allometric growth can reflect changes needed for reproduction, feeding behaviors, or habitat use [3].
The studies on biological characteristics of the Mekong fish have been limited, except for some data on Glossogobius giuris, Butis koilomatodon, A. vachellii, Butis butis, St. dubiosus [15, 16, 17, 18, 19]. Some works are devoted to the reproduction of fish – B. koilomatodon, Periophthalmodon septemradiatus, Stigmatogobius pleurostigma, Trypauchen vagina, Boleophthalmus boddarti [18, 20, 21, 22, 23].
LWR and CF are important biological characteristics of fish health. Nevertheless, at present, studies devoted to the study of this issue of fish in the Mekong Delta are not numerous. For Stigmatogobius pleurostigma and Johnius borneensis season dynamics of these parameters were shown by Dinh, (2017) [24] and Solania & Seronay (2017) [25] respectively. The LWR, CF, and gut contents of Syncrossus helodes and Yasuhikotakia modesta from the Mekong River (Northeastern Thailand) were described by Hanjavanit (2013) [26]. The LWR of Boleophthalmus boddarti was W=0.006TL3.13. As in some of the fish in our research, the values of b of females were higher than that of males – 3.57 and 2.94, respectively [27].
Mekong River Commission (MRC) presented values of a and b for 11 fish species (Botia helodes, Cosmochilus harmand, Cynoglossus microlepi, Gyrinocheilus pennocki, Henicorhynchus lobatus, H. siamensis, Hypsibarbus malcolmi, Pangasianodon gigas, Pangasius conchophilus, Paralaubuca typus, Probarbus jullieni, and one shrimp Macrobrachium sp. (probably M. rosenbergi). The b-values ranged from 2.36 for Paralaubuca typus to 3.32 for Probarbus jullien. The average b was 3.16 [28].
Pin et al. (2020) analyzed LWR from 23,408 individuals of 45 species in 11 families, 4 orders. As a result, 27 species (60.00%) had negative allometries (b < 3), 3 species (6.67%) isometries (b = 3), and 15 species (33.33%) positive allometries (b > 3) [29]. One of the fish presented in the article is common with our research – P. wolffii. Its parameters: Lmin – 2.60cm, Lmax – 25.00 cm, Lmean – 8.79 cm, Wmin – 1.80g, Wmax – 165.20 g, Wmean 12.61, a-value – 0.79, b-value – 1.20. These values are significantly lower than ours, especially the extremely low value of b. Apparently, This difference may be attributed to the fact that the authors included a high proportion of immature individuals in their study, Whereas our study focused solely on mature specimens. To compare our studies, we calculated the values of a and b considering immature individuals (the number of immature individuals in the total sample was 22%). The new LWR for P. wolffii is W=0.015L2.99. Thus, the values of a and b did not decrease but rather increased on the contrary. We decided to test our assumption that "an increase in the number of immatures decreases the b value" for another species in whose catches there were more immature species (P. melanochir – 74% of immatures). Some decrease in b values was noted (obtained LWR is W=0.006L2.99). Another possible reason for low b values is seasonal changes (food availability migration, spawning). For J. borneensis and Stigmatogobius pleurostigma b values have been reported to vary widely, ranging from 0.35 to 3.46 [30] and from 2.18 to 3.21 [24] respectively.
The condition factor (CF) serves as a morphometric index used to evaluate the physiological status of fish, based on the principle that individuals of a given length with greater mass are in better 'condition' [3, 31]. CF is significant for monitoring feeding intensity, age, and growth. Fish with higher CF values are considered to be in better condition than those with lower values [25]. In our study, all species had relatively high CF values, except for both Coilia species. However, this interpretation should be approached cautiously, given the environmental context of the Mekong Delta. The region faces challenges such as pollution, overfishing, and the prevalent capture of smaller-sized fish. These factors could influence the condition and overall health of fish populations, suggesting that further monitoring and more comprehensive assessments are needed to confirm these findings.
4. CONCLUSIONS
The length-weight relationships of 16 fish species in the Mekong Delta, Vietnam was estimated with the slope parameter range from 2.72 to 3.28, of which eight species exhibited positive allometry, seven species demonstrated negative allometry, and one species showed isometric growth. Fulton’s condition factor for the 16 fish species was relatively high, ranging from 0.28 to 2.09. The obtained data can be used for further research aimed at providing practical recommendations for the conservation of biodiversity and for regulating fisheries.
Acknowledgment: This investigation was financially supported by the Joint Vietnam-Russia Tropical Science and Technology Research Center under grant No. Ecolan 3.4-3 – “The Mekong Delta Ecosystem in the Conditions of the Global Climate Changes and Anthropogenic Impact” and as in part of the framework of IBSS state research assignment under grant No. 124022400148-4 - “Biodiversity as the basis for the sustainable functioning of marine ecosystems, criteria and scientific principles for its conservation.
Tài liệu tham khảo
2. D. D. Tran et al., Fishes of the Mekong Delta, Vietnam, Can Tho: Can Tho University Publishing House, 2013, 147 p.
3. R. Froese, Cube law, condition factor and weight-length relationships: History, meta-analysis and recommendations, Journal of Applied Ichthyology, Vol. 22, No. 4, pp. 241-253, 2006. DOI: 10.1111/j.1439-0426.2006.00805.x
4. A. S. Halls, I. et al., Atlas of deep pools in the Lower Mekong River and some of its tributaries, MRC Technical Paper, No. 31, Phnom Penh, Cambodia, Mekong River Commission, 2013, 69 p.
5. V. N. Ut, A. V. Hoa and H. P. Vinh, Status of fish biodiversity and fishing on Hau River, Mekong Delta, Vietnam, Annales de Limnologie - International Journal of Limnology, Vol. 56, No. 14, 11 p. 2020. DOI: 10.1051/limn/2020012
6. E. P. Karpova, et al., Spatial Variations in Fish Abundance in the Mekong Delta, Russian Journal of Ecology, Vol. 52, pp. 146–154, 2021. DOI: 10.1134/S1067413620050082
7. E. P. Karpova, E. R. Ablyazov, S. V. Kurshakov, I. I. Chesnokova, C. N. Dinh and T. B. Hai, Distribution, population structure, and selected biological characteristics of fish in the genus Coilia (Engraulidae) in the Mekong Delta, Journal of Ichthyology, Vol. 61, pp. 554–563, 2021. DOI: 10.1134/S003294522104007X.
8. E. P. Karpova et al., Distribution of fish and decapodes in the Mekong delta (Vietnam), Kỷ yếu Hội nghị khoa học Kỷ niệm 30 năm ngày truyền thống Chi nhánh Phía Nam (20/02/1992-20/02/2022). Hà Nội – Nha Trang - TP. Hồ Chí Minh, Tháng 12 năm 2021 [Hồ Chí Minh]: Trung tâm Nhiệt đới Việt – Nga, Chi nhánh Phía Nam, 2021, tr. 375–382.
9. W. J. Rainboth, Fishes of the Cambodian Mekong. FAO species identification field guide for fishery purposes, Rome: FAO, 1996, 265 p.
10. W. J. Rainboth, C. Vidthayanon and Đ. Y. Mai, Fishes of the Greater Mekong ecosystem with species list and photographic atlas. Museum of Zoology, University of Michigan, USA 2012, 173 p.
11. M. Kottelat, The Fishes of the inland waters of Southeast Asia: A Catalogue and core bibliography of the fishes known to occur in freshwaters, mangroves and estuaries, The Raffles Bulletin of Zoology, Vol. 27, pp. 1–663, 2013.
12. Y. Taki et al., Fishes of the Indochinese Mekong, Tokyo: Nagao Natural Environment Foundation, 2021, 562 p.
13. R. Froese, D. Pauly Eds., FishBase. World Wide Web electronic publication, 2021. [Online]. Available: http://www.fishbase.org. [Accessed Dec. 10, 2024].
14. E. D. Le Cren, The length-weight relationship and seasonal cycle in gonad weight and condition in the perch (Perca flavescens), Journal of Animal Ecology, Vol. 20, pp. 201–219, 1951. DOI: 10.2307/1540
15. Q. M. Dinh, The length-weight relationship of the duckbill sleeper Butis butis (Hamilton, 1822), The University of Danang - Journal of Science and Technology, Vol. 3(112), pp. 47–49, 2017b.
16. Q. M. Dinh, Y. N. Phan and D. D. Tran, Population biology of the goby glossogobius giuris (Hamilton, 1822) caught in the Mekong Delta, Vietnam, Asian Fisheries Science, Vol. 30, Iss. 1, pp. 26–37, 2017.
17. Q. M. Dinh, T. V. Nguyen and D. D. Tran, Population biological parameters of Ambassis vachellii (Perciformes: Ambassidae) caught from Bay Hap and Cua Lon estuaries, Ca Mau province, Vietnam, Egyptian Journal of Aquatic Biology and Fisheries, Vol. 24, Iss. 7, pp. 779–801, 2020a. DOI: 10.21608/ejabf.2020.132409.
18. Q. M. Dinh, L. T. Tran, N. C. Ngo, T. B. Pham and T. T. K. Nguyen, Reproductive biology of the unique mudskipper periophthalmodon septemradiatus living from estuary to upstream of the Hau River, Acta Zoologica, Vol. 101. Iss. 2, pp. 206–217, 2020b. DOI: 10.1111/azo.12286.
19. D. D. Tran, V. T. Nguyen and Q. M. Dinh, Population dynamics of Stolephorus dubiosus in Bay Hap and Cua Lon estuaries, Mekong Delta, Vietnam, AACL Bioflux, Vol. 13, Iss. 4, pp. 2250–2264, 2020.
20. M. Q. Dinh, Aspects of th reproductive biology of the red goby Trypauchen vagina (Gobiidae) from the Mekong Delta, Journal of Applied Ichthyology, Vol. 34, No. 1, pp.103-110, 2017. DOI: 10.1111/jai.13521
21. Q. M. Dinh, T. T. N. Tran, Reproductive biological traits of the goby Stigmatogobius pleurostigma (Bleeker, 1849) from the Mekong Delta, Vietnam, Indian Journal of Fisheries, Vol. 65, No. 1, pp. 20–25, 2018. DOI: 10.21077/ijf.2018.65.1.68188-04
22. Q. M. Dinh, T. T. G. Nguyen and T.K.T. Nguyen, Reproductive biology of the mudskipper Boleophthalmus boddarti in Soc Trang, Academia Journal of Biology, Vol. 37, No. 3, pp. 362–369, 2015. DOI: 10.15625/0866-7160/v37n3.6720.
23. Q. M. Dinh, T. T. H. Lam, T. H. D. Nguyen, T. M. Nguyen, T. T. K. Nguyen and N. T. Nguyen, First reference on reproductive biology of Butis koilomatodon in Mekong Delta, Vietnam, BMC Zoology, Vol. 6, No. 7, 2021. DOI: 10.1186/s40850-021-00072-y
24. Q. M. Dinh, Morphometrics and condition factor dynamics of the Goby stigmatogobius pleurostigma (Bleeker 1849) during dry and wet seasons in the Mekong Delta, Vietnam, Asian Fisheries Science, Vol. 30, pp. 17–25, 2017a. DOI: 10.33997/j.afs.2017.30.1.002.
25. C. L. Solania, R. A. Seronay, Reproductive aspect of the native Sharpnose hammer croaker, Johnius borneensis (Bleeker, 1850) from Agusan River Estuary, Caraga region, Philippines, Journal of Entomology and Zoology Studies, Vol. 5, No. 5, pp. 220–228, 2017. DOI: 10.21077/ijf.2018.65.1.68188-04
26. C. Hanjavanit, S. Buromra and N. Sangpradub, The length-weight relationships, condition factors and gut contents of Syncrossus helodes (Sauvage, 1876) and Yasuhikotakia modesta (Bleeker, 1864) from the Mekong River, Muang District, Nong Khai Province, Northeastern Thailand, African Journal of Agricultural Research, Vol. 8, pp. 5508–5517, 2013. DOI: 10.5897/AJAR12.2120.
27. Q. M. Dinh, A preliminary study on length-weight relationship of the mudskipper Boleophthalmus boddarti in Soc Trang, Academia Journal of Biology, Vol. 36, No. 1, pp. 88–92, 2014. DOI: 10.15625/0866-7160/v36n1.4524.
28. A. S. Halls, M. Kshatriya, Modelling the cumulative effects of mainstream hydropower dams on migratory fish populations in the lower Mekong Basin, MRC Technical Paper, No. 25, Mekong River Commission, Vientiane, 2009, 104 p. DOI:
10.52107/mrc.ajhz5g
29. K. Pin et al., Cambodian freshwater fish assemblage structure and distribution patterns: Using a large-Scale monitoring network to understand the dynamics and management implications of species clusters in a global biodiversity hotspot, Water, Vol. 12, Iss. 9, 2506, 2020. DOI: 10.3390/w12092506.
30. S. M. Tagarao, C. L. Solania, J. C. Jumawan, S. G. Masangcay and L. B. Calagui, Length-Weight Relationship (LWR), Gonadosomatic Index (GSI) and Fecundity of Johnius borneensis (Bleeker, 1850) from Lower Agusan River basin, Butuan City, Philippines, Journal of Aquaculture Research and Development, Vol. 11, 598, 2020. DOI: 10.35248/2155-9546.20.11.598.
31. K. M. Konan, L. Doumbia, A. B. Adépo-Gourène, A. Ouattara and G. Gourène, Relationships between morphometric characteristics of brackishwater prawn, Macrobrachium macrobrachion (Herklots, 1851), of Côte d’Ivoire (West Africa), Iranian Journal of Fisheries Sciences, Vol. 16, Iss. 1, pp. 275–295, 2017.