PROTECTIVE PERFORMANCE OF THE MIGRATING CORROSION INHIBITOR MCI-VN FOR STEEL REINFORCEMENT IN CONCRETE PORE SOLUTION
Coastal Branch, Joint Vietnam - Russia Tropical Science and Technology Research Center
30 Nguyen Thien Thuat, Nha Trang, Khanh Hoa, Vietnam
Phone: 0862452609; Email: cnlinh0812@gmail.com
Main Article Content
Abstract
A blended corrosion inhibitor (MCI-VN) based on a synergistic combination of inorganic and organic salts with a nonionic surfactant is proposed to protect steel reinforcement in concrete under chloride exposure. The composition is suitable for use as an admixture in the production of new reinforced concrete structures or repair mortars, as well as for surface application to existing structures (migrating corrosion inhibitor). This study evaluates the anti-corrosion properties of the inhibitor in the concrete pore solution. The assessment was performed using electrochemical methods (potentiodynamic polarization and impedance spectroscopy) and gravimetric analysis. As the inhibitor concentration (Cinh) increased in the 0.25-5.0 wt.% range, the inhibition efficiency rose to 92-97%, depending on the evaluation method. During 30-day gravimetric experiments, complete suppression of corrosion was observed at Cinh ≥ 2.0 wt.%. Comparison with the commercial inhibitor IFKhAN-80 showed comparable or superior protective performance under equivalent conditions. Therefore, MCI-VN can be considered an effective solution for improving the corrosion resistance of reinforced concrete structures in chloride-containing environments.
Keywords
Reinforcing steel, corrosion inhibitors, chlorides, reinforced concrete, concrete pore solution.
Article Details
- Highlights:
A blended corrosion inhibitor (MCI-VN) based on inorganic and organic salts with a nonionic surfactant was developed for reinforcing steel in chloride-containing concrete environments.
Electrochemical tests (potentiodynamic polarization and impedance spectroscopy) in concrete pore solution with 3% NaCl showed 92–97% inhibition efficiencies at concentrations of 2.0-5.0 wt.%.
Gravimetric experiments over 30 days demonstrated complete corrosion suppression at Cinh ≥ 2.0 wt.% for MCI-VN, while IFKhAN-80 required Cinh ≥ 3.0 wt.% to achieve the same effect.
Comparative evaluation confirmed that MCI-VN exhibited slightly higher protective performance than the commercial inhibitor IFKhAN-80 under equivalent conditions.
1. INTRODUCTION
Reinforced concrete is one of the most widely used materials in civil and industrial infrastructure construction. Reinforced concrete structures are degraded when exposed to environmental factors containing aggressive agents (such as chlorides, carbon dioxide, sulfates, acids, etc.) [1]. The financial costs associated with maintaining the integrity and safety of reinforced concrete (RC) structures were estimated at 18–21 billion USD annually in the United States as of 2014 [2]. Indirect costs, including traffic disruptions and reduced operational efficiency, can exceed direct expenses by up to tenfold [3].
Chloride-induced corrosion of steel reinforcement is the primary cause of reinforced concrete deterioration [4, 5]. This issue is particularly relevant for marine infrastructure (e.g., ports, hydraulic structures), facilities in the chemical industry (e.g., salt extraction, fertilizer production), and transportation structures located near coastlines or in regions where deicing treatments are applied during winter. A distinguishing feature of chloride-induced corrosion in concrete is its localized nature [6]. Pitting and crevice corrosion can develop rapidly, leading to partial or complete structural failure. This form of corrosion is more difficult to detect than uniform corrosion, such as that caused by concrete carbonation [7].
Various methods have been developed to enhance the durability of concrete structures. One practical approach for protecting steel reinforcement from corrosion is using inhibitors [8-10]. These compounds can be introduced into the concrete mix during construction or into repair mortars (so-called admixture inhibitors) or applied to the surface of existing structures in the form of migrating corrosion inhibitors (MCIs) [11]. A literature review indicates that one of the most effective classes of corrosion inhibitors comprises blended formulations [12]. These typically contain organic and inorganic components and additional substances designed for application as MCIs. One such commercially available inhibitor is IFKhAN-80 (TU 2458-005-66126292-11, IPCE RAS, Russia) [13].
In Vietnam, chloride-induced corrosion has emerged as a critical durability issue for reinforced concrete infrastructure. Field investigations of coastal piled-pier port structures have revealed significant rebar corrosion and structural damage occurring as early as 10–15 years into service, with widespread deterioration commonly observed within 20–25 years [14]. In light of the absence of domestically produced migrating corrosion inhibitors, developing a locally manufactured solution is essential to ensure cost-effective and accessible corrosion protection for national infrastructure.
Accordingly, a novel corrosion inhibitor composition (MCI-VN) has been developed as either an admixture in fresh concrete or repair formulations or a migrating corrosion inhibitor. The MCI-VN formulation is based on a synergistic combination of inorganic and organic salts with a nonionic surfactant. This study aims to evaluate the efficacy of the migrating corrosion inhibitor MCI-VN in model aqueous solutions to protect reinforcing steel in the presence of chlorides and to compare its anti-corrosion performance with that of the commercial product IFKhAN-80.
2. MATERIALS AND METHODS
2.1. Materials
The MCI-VN formulation was prepared by blending the following components (by weight): sodium hydroxide (NaOH) - 1.73 g, benzoic acid (C6H5COOH) - 5.31 g, sodium nitrite (NaNO2) - 20.97 g, glycerol - 1.00 g, nonionic surfactant (cocamide diethanolamine) - 0.50 g, diethanolamine (DEA) - 0.50 g. The remaining portion of the 100 g formulation consisted of deionized water. This mixture was homogenized until the complete dissolution of all components was achieved, and it was used directly in corrosion testing.
Smooth-surfaced reinforcing steel of grade CB300-T, conforming to TCVN 1651-1:2018, with a diameter of 10 mm, was used as the test material. The elemental composition of the steel, determined according to ASTM E415:2021, was (wt.%): Fe 98.518, C 0.236, Mn 0.413, Si 0.155, P 0.012, S 0.044, Ni 0.131, Cr 0.035, Cu 0.334, Mo 0.010, V 0.002, Ti 0.001, Al 0.016, Nb 0.001, W 0.005, Sn 0.037, Co 0.009, Pb 0.019, and Zn 0.023. For the potentiodynamic and impedance spectroscopy measurements, one end of each steel rod was soldered to an insulated copper wire, and the soldered area was coated with epoxy resin.
The concrete pore solution was prepared by extracting crushed mortar with single-distilled water for 24 hours. The mass ratio of water to solid material was maintained at 1:10. The resulting solution was filtered through folded paper filters. The mortar was prepared from a cement-sand mixture consisting of PCB40 Portland cement (Nghi Son), whose composition (wt.%) was SiO2 22.0, Al2O3 8.0, CaO 62.0, Fe2O3 3.0, MgO 2.1, SO3 2.1, K2O 0.6, and Na2O 0.2, and dry, washed quartz sand with a particle size range of 0.20-0.63 mm. The cement-to-sand mass ratio was 1:2, and the water-to-cement ratio was set at w/c = 0.4.
2.2. Methods
A combination of electrochemical and gravimetric measurements was employed to evaluate the protective performance of the proposed formulation and the commercial inhibitor IFKhAN-80.
The surface of the steel electrodes was polished using P1000 sandpaper (Al2O3 abrasive with a particle size of 14-20 µm) to a cleanliness grade of St3 following ISO 8501-1:2014 [15] and then degreased with 96% ethanol.
Potentiodynamic polarization curves were recorded in a concrete pore solution with the addition of 3% NaCl. The corrosion current density (icor) was calculated using the linear polarization resistance method proposed by F. Mansfeld [16]. A three-electrode glass cell was used, with the working, counter, and reference electrodes in a single compartment under natural aeration. The working electrode was positioned such that the thickness of the solution above its surface was maintained at 20 ± 2 mm. A platinum mesh served as the counter electrode. The silver/silver chloride (Ag/AgCl) reference electrode was placed in a separate vessel connected to the cell via a salt bridge made of agar-agar gel containing KNO3 to prevent chloride contamination of the test solution. The reference electrode potential was +201 mV versus the standard hydrogen electrode (SHE). Polarization curves were recorded using a potentiostat/galvanostat (Autolab PGSTAT, Netherlands) after stabilizing the open circuit potential (Ecor) for 60 minutes. The scan rate was 0.2 mV/s. The working electrode potential (E) is reported versus the Ag/AgCl scale, and the current density (i) was calculated for the geometric surface area of the working electrode, which ranged from 7.0 to 8.5 cm2, depending on the experiment.
The ability of the investigated formulations to reduce the corrosion rate was evaluated based on the inhibition efficiency, which was calculated using the following equation:
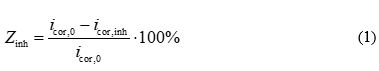
where icor,0 and icor,inh are the corrosion current densities in the blank (control) experiment and the presence of the inhibitor, respectively.
Electrochemical impedance spectra were recorded using a potentiostat/galvanostat (Autolab PGSTAT, Netherlands). After stabilizing the open circuit potential (Ecor) for 60 minutes, the frequency response was measured from 0.01 Hz to 50 kHz under open-circuit (zero-current) conditions.
The analysis of the frequency dependence, selection of the equivalent electrical circuit, and determination of the nominal values of its elements were performed using the DCS software package. The results were presented in the form of Nyquist plots. The inhibition efficiency (ηinh) was calculated using the following equation:
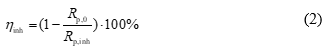
where Rp,0 and Rp,inh are the polarization resistances in the control experiment and the presence of the inhibitor, respectively.
Corrosion-induced mass loss was monitored over 30 days using unstressed (non-polarized) steel specimens. Corrosion products were removed following ISO 8407:2021 [17]. The corrosion inhibition efficiency of the tested formulations was also evaluated based on mass loss data using the following equation:
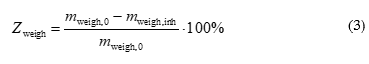
where mweigh,0 and mweigh,inh are the corrosion rates determined from mass loss measurements in the control experiment and the presence of the inhibitor, respectively. Visual inspection of the surface of the reinforcing steel specimens was also carried out.
3. RESULTS
Introducing MCI-VN into the concrete pore solution containing 3% NaCl resulted in a shift of the corrosion potential (Ecor) in the anodic direction. As the inhibitor concentration (Cinh) increased from 0.25 wt.% to 5.0 wt.%, the magnitude of this shift increased from 50 to 180 mV (Table 3). Increasing the concentration of MCI-VN also reduced current densities in both the anodic and cathodic branches of the polarization curves (Figure 1b).
A similar anodic shift in Ecor (from 10 to 160 mV) and a decrease in current densities with increasing inhibitor concentration were observed for IFKhAN-80 (Figure 1a). Notably, the polarization curves were nearly identical in the range of Cinh = 3.0-5.0 wt.%.
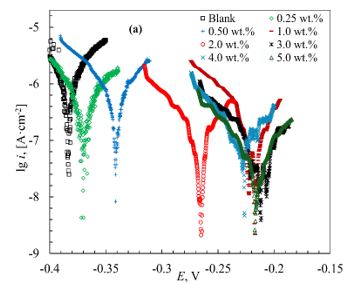
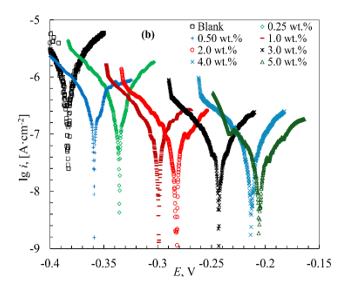
Figure 1. Initial segments of polarization curves of reinforcing steel in concrete pore solution + 3% NaCl with varying concentrations of inhibitors: IFKhAN-80 (a) and MCI-VN (b)
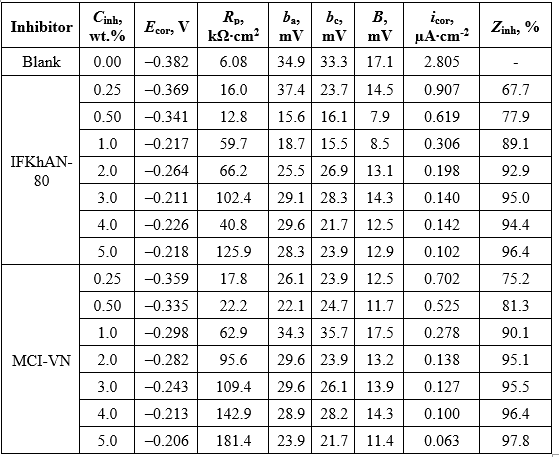
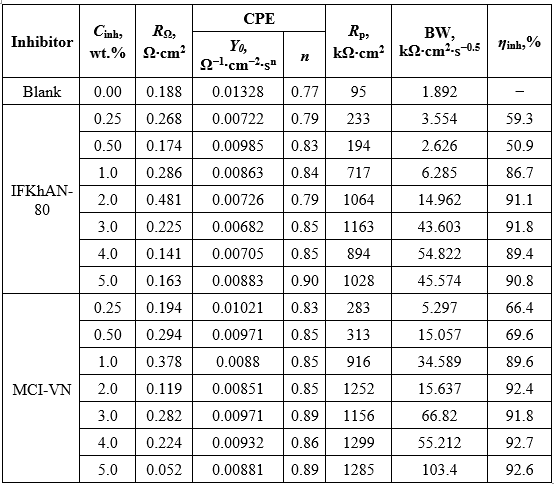
The calculated values of icor are presented in Table 1. In general, an increase in the inhibition efficiency (Zinh) was observed with increasing concentration of the tested formulations (Table 1). The highest Zinh value of 97.8% for MCI-VN was achieved at Cinh = 5.0 wt.%, while the maximum for IFKhAN-80 was 96.4%.
Table 1. Corrosion parameters of reinforcing steel in concrete pore solution with 3% NaCl in the presence of inhibitors, calculated using the Mansfeld method
The Nyquist plots in the absence and presence of the inhibitors showed similar shapes, featuring a distorted semicircle with varying diameters and a linear portion at low frequencies (Figure 2). The impedance spectra in the presence of inhibitors significantly differed from the control experiment at all concentrations. As the inhibitor concentration (Cinh) increased for both IFKhAN-80 and MCI-VN, the diameter of the semicircles also increased. The largest radii were observed at Cinh ≥ 1.0 wt.%.
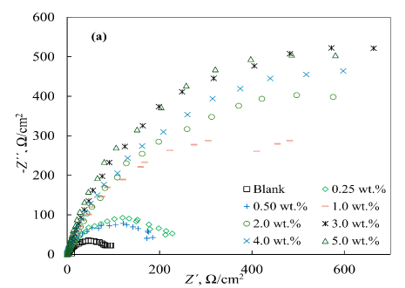
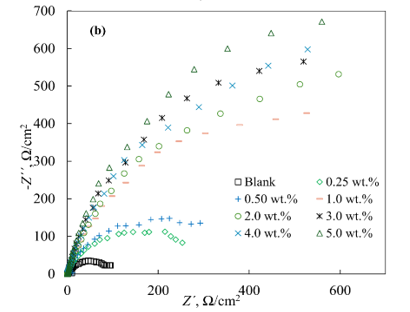
Figure 2. Nyquist plots for reinforcing steel in concrete pore solution with 3% NaCl and varying concentrations of inhibitors: IFKhAN-80 (a) and MCI-VN (b)
The plots are satisfactorily described by the equivalent circuit shown in Figure 3.
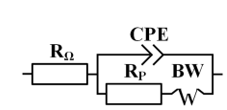
Figure 3. The equivalent circuit used to analyze electrochemical impedance spectra, where RΩ - solution ohmic resistance, CPE - constant phase element, Rp - polarization resistance of the electrochemical reaction, BW - Warburg impedance.
The calculated values of the equivalent circuit elements are shown in Table 2. At Cinh ≥ 1.0 wt.%, the inhibition efficiencies (ηinh) for both tested formulations exceeded 85%.
Table 2. Elements of the equivalent circuit for reinforcing steel in concrete pore solution with 3% NaCl in the presence of inhibitors
The appearance of the steel specimens at the end of the exposure period is shown in Figure 4. In the control experiment, multiple corrosion defects - such as stains, clusters of pits, and localized corrosion - were observed on the metal surface, visible to the naked eye. No corrosion products or pitting were detected for the IFKhAN-80 inhibitor at Cinh ≥ 3.0 wt.%. At lower concentrations, isolated or grouped pits with diameters up to 3 mm were observed. Adding MCI-VN at Cinh ≤ 1.0 wt.% resulted in forming 1-2 pits with diameters ranging from 3 to 5 mm. With a further increase in concentration to Cinh ≥ 2.0 wt.%, no signs of corrosion damage were detected on the rebar surface, which retained a metallic sheen.

Figure 4. The general appearance of reinforcing steel samples after 30 days of exposure in concrete pore solution with 3% NaCl without inhibitor (0) and with various concentrations of IFKhAN-80 (1) and MCI-VN (2). Inhibitor concentrations (wt.%) are indicated in the images.
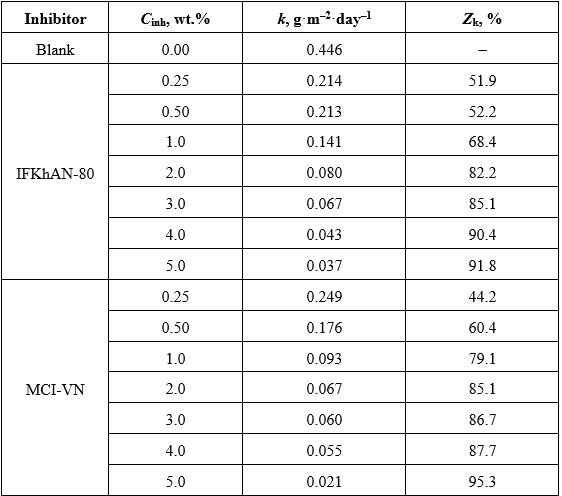
The mass loss measurements are shown in Table 3. When the inhibition efficiency based on weight loss (Zk) exceeded 85%, no pitting or corrosion products were observed on the steel surface. For MCI-VN, this corresponds to Cinh ≥ 2.0 wt.%, whereas for IFKhAN-80, the same result was achieved at Cinh ≥ 3.0 wt.%.
Table 3. Results of gravimetric measurements over 30 days
4. DISCUSSION
The results indicate that MCI-VN and IFKhAN-80 exhibit strong anti-corrosion performance at Cinh ≥ 1.0-2.0 wt.% concentrations, where Zinh exceeds 90% [1]. The anodic shift in Ecor with increasing inhibitor concentration suggests a predominant influence on the anodic partial reaction of steel oxidation [18]. The reduction in current densities in both anodic and cathodic branches of the polarization curves for both inhibitors further supports their effectiveness in mitigating corrosion processes.
The Nyquist plots (Figure 2) and the equivalent circuit analysis (Figure 3, Table 2) confirm that the impedance spectra in the presence of inhibitors significantly differ from the control experiment, with larger semicircle diameters at higher concentrations indicating enhanced corrosion resistance. The inhibition efficiencies (ηinh) calculated from impedance spectroscopy align with those from potentiodynamic measurements, reinforcing the reliability of the results. MCI-VN consistently showed ηinh values 1-3% higher than IFKhAN-80 at equivalent concentrations.
Visual inspection and mass loss measurements (Figure 4, Table 3) corroborate the electrochemical findings. The absence of corrosion defects at Cinh ≥ 2.0 wt.% for MCI-VN and Cinh ≥ 3.0 wt.% for IFKhAN-80 highlights MCI-VN’s superior performance at lower concentrations. The lack of a clear correlation between the number or size of corrosion defects and inhibitor concentration at lower Cinh values suggests that a threshold concentration is necessary for complete corrosion suppression.
Based on single-point measurements of corrosion rate using the polarization resistance method, electrochemical impedance spectroscopy, and long-term mass loss experiments in concrete pore solution with 3% NaCl, it was reliably established that the proposed blended inhibitor MCI-VN exhibits strong inhibitory performance toward reinforcing steel at concentrations of Cinh ≥ 2.0 wt.%. The synergistic combination of inorganic and organic salts with a nonionic surfactant in MCI-VN likely contributes to its enhanced performance compared to IFKhAN-80, particularly at lower concentrations.
5. CONCLUSION
A blended corrosion inhibitor (MCI-VN) was developed and evaluated for protecting reinforcing steel in concrete under chloride exposure. The components of the inhibitor allow it to be used as an admixture in new concrete construction, as a component in repair mortars, or as a surface-applied migrating corrosion inhibitor (MCI) for existing structures. Investigations were carried out in a model pore solution containing 3% NaCl. The high anti-corrosion performance of MCI-VN was demonstrated based on polarization resistance measurements, electrochemical impedance spectroscopy, and gravimetric tests. The highest inhibition efficiency, ranging from 85% to 97% depending on the evaluation method, was achieved at concentrations of Cinh > = 2.0-5.0 wt.%.
Comparison with the commercial inhibitor IFKhAN-80 showed comparable results; however, the efficiency of MCI-VN was up to 3% higher. It was also observed that in the case of MCI-VN, no signs of corrosion were detected on the surface of steel reinforcement after 30 days of exposure at Cinh ≥ 2.0 wt.%, while for IFKhAN-80, a similar outcome was achieved only at Cinh ≥ 3.0 wt.%. These findings suggest that MCI-VN is a promising candidate for enhancing the durability of reinforced concrete structures exposed to chloride environments.
Acknowledgments: This work was supported by the Coastal Branch of the Joint Vietnam-Russia Tropical Science and Technology Research Center (VB.Đ1.11/23).
Author contributions: Cao Nhat Linh: Conceptualization. Nguyen Van Chi, Nguyen Duc Anh: Methodology. Cao Nhat Linh, Nguyen Duc Anh, Shevtsov D.S.: Formal analysis and investigation. Cao Nhat Linh, Nguyen Van Chi: Writing - original draft preparation. Le Thi My Hiep, Nong Quoc Quang: Writing - review and editing. All authors have read and agreed to the published version of the manuscript.
Conflict of interest statement: The authors declare that there are no conflicts of interest related to this article.
References
2. M. Shen, A. Furman and A. Hansen, Protecting concrete reinforcement using admixture with migrating corrosion inhibitor and water repellent component in NACE CORROSION - NACE, 2014, p. 4250.
3. D. Trejo, C. Halmen and K. Reinschmidt, Corrosion performance tests for reinforcing steel in concrete: technical report №. FHWA/TX-09/0-4825. Texas, Texas Transportation Institute, 2000.
4. K. Osterminski, To fully probabilistic modeling of steel corrosion in concrete: A contribution to the durability design of reinforced concrete components, Doctoral Dissertation, Technical University of Munich, Munich, Germany, 2013, 211 p.
5. U. Angst, B. Elsener, C. K. Larsen and Ø. Vennesland, Critical chloride content in reinforced concrete - A review, Cem. Concr. Res., Vol. 39, pp. 1122-1138, 2009. DOI: 10.1016/j.cemconres.2009.08.006
6. С. Alonso, M. Castellote and C. Andrade, Chloride threshold dependence of pitting potential of reinforcements, Electrochimica Acta, Vol. 47, No. 21, pp. 3469-3481, 2002. DOI: 10.1016/S0013-4686(02)00283-9
7. K. Tuutti, Corrosion of steel in concrete. Stockholm, Cementoch betonginst, 1982, 469 p.
8. A. Goyal, H. S. Pouya, E. Ganjian and P. Claisse, A review of corrosion and protection of steel in concrete, Arab. J. Sci. Eng, Vol. 43, pp. 5035-5055, 2018. DOI: 10.1007/s13369-018-3303-2
9. M. Ormellese, M. Berra, F. Bolzoni and T. Pastore, Corrosion inhibitors for chlorides induced corrosion in reinforced concrete structures, Cem. Concr. Res., Vol. 36, No. 3, pp. 536-547, 2006. DOI: 10.1016/j.cemconres.2005.09.003
10. J. S. Reoua, K. Y. Ann, The electrochemical assessment of corrosion inhibitor effect of calcium nitrite in blended concretes, Mater. Chem. Phys. Vol. 109, pp. 526-533, 2008. DOI: 10.1016/S0254-0584(08)00060
11. M. Ormellese, F. Bolzoni, S. Goidanich and M. P. Pedeferri, A. Brenna, Corrosion inhibitors in reinforced concrete structures Part 3-migration of inhibitors into concrete, Cor. Eng., Sci. Tech., Vol. 46, No. 4, pp. 334-339, 2011.
12. N. L. Cao et al., Migrating corrosion inhibitors for steel reinforcement in concrete under chloride activation: A review, Int. J. Corros. Scale Inhib., Vol. 14, No. 2, pp. 469-490, 2025. DOI: 10.17675/2305-6894-2025-14-2-4
13. N. N. Andreev, I. A. Gedvillo, A. S. Zhmakina, D. S. Bulgakov and S. S. Vesely, Environmental testing of the efficiency of IFKhAN-80, an inhibitor for corrosion protection of steel reinforcement in concrete, Int. J. Corros. Scale Inhib, Vol. 5, No. 4, pp. 319-324, 2016. DOI: 10.17675/2305-6894-2016-5-4-2
14. T. B. D. Nguyen, J. Huh, T. T. Vu, M. L. Tran and V. H. Mac, Development of Indicator for Piled Pier Health Evaluation in Vietnam Using Impact Vibration Test Approach, Buildings, Vol. 14, p. 2366, 2024. DOI: 10.3390/buildings14082366
15. ISO 8501-1-2014. Preparation of steel substrates before application of paints and related products - Visual assessment of surface cleanliness. Part 1: Rust grades and preparation grades of uncoated steel substrates and of steel substrates after overall removal of previous coatings.
16. F. Mansfeld, Tafel slopes and corrosion rates obtained in the pre-Tafel region of polarization curves, Cor. Sci., Vol. 47, pp. 3178-3186, 2005. DOI: 10.1016/j.corsci.2005.04.012
17. ISO 8407:2021. Corrosion of metals and alloys - Removal of corrosion products from corrosion test specimens.
18. N. D. Nam, P. Van Hien, N. T. Hoai and V. T. H. Thu, A study on the mixed corrosion inhibitor with a dominant cathodic inhibitor for mild steel in aqueous chloride solution, J Taiwan Instit. Chem. Eng., Vol. 91, pp. 556-569, 2018. DOI: DOI: 10.1016/j.jtice.2018.05.041